
-
NEWS最新消息

- 本公司核准成為股票公開發行,股票代號「7759」。
- 2024/6/14
- 狂賀! 治療乾眼症核酸新藥,臨床二期試驗解盲成功,有效改善乾眼症引起的角膜受損、 並增加病人的舒適感,且安全性良好。
- 2024/4/9
- 治療乾燥症患者角膜破損核酸新藥,獲得臺灣FDA核准展開臨床二期試驗。
- 2024/4/9
- 睛航生醫於民國113年3月7日與視航生醫合併完後,視航生醫為存續公司。
- 2024/3/14
- 治療乾眼症核酸新藥,獲得臺灣FDA核準展開臨床二期試驗
- 2023/9/6
- 專利獲證: 治療乾眼症眼藥水獲得中華民國專利,專利號碼I796875
- 2023/3/21
- 治療乾眼症核酸新藥,獲得泰國FDA核準展開臨床二期試驗
- 2023/1/5
- 治療乾眼症核酸新藥,獲澳洲核準展開臨床二期試驗
- 2022/10/5
- 委託勤業眾信進行財稅簽
- 2022/9/27
- 獲得 "2022台北生技獎”
- 2022/7/7
- 台灣衛福部食藥署於2022年4月進行第一期臨床試驗GCP查核,查核結果順利,無任何缺失。
- 2022/5/31
- 視航生醫於2021年完成臨床一期試驗。
- 2022/1/30
- 專利獲證:治療近視眼藥水獲得中國大陸專利,專利號碼CN107922948B
- 2021/7/6
- 狂賀! 美國食品藥物管理局(FDA)無異議並且提前核准執行臨床試驗(IND)
- 2021/2/26
- 獲得歐盟專利 - 治療近視的藥物組合及運用
- 2020/11/13
- 網路詐騙治療近視眼藥水
- 2020/7/31
- 榮獲2020經濟部“破殼而出”企業獎
- 2020/10/20
- 獲得行政院科技部 選出3位最成功教授創業家影片
- 2020/2/10
- 治療近視眼藥水獲得韓國專利,專利號碼10-2048165
- 2019/12/9
- 治療近視眼藥水獲得日本專利,專利號碼6516196
- 2019/4/26
- 治療近視眼藥水獲得美國專利,專利號碼US010179913B2
- 2019/1/15
- 治療近視眼藥水獲得台灣專利,專利號碼I585205
- 2017/6/1


-
ABOUTSUNHAWK VISION公司簡介

視航生物醫學股份有限公司(視航生醫)
視航生物醫學股份有限公司之設立,
源於科技部之生技整合育成中心(Si2C)
及價創計畫積極輔導以及補助的抗近視新藥開發,
是開發嶄新RNA治療眼藥水,以治療兒童近視為目標。
2019年8月研發團隊將此技術及專利由任教之學校技轉,
並且引進民間資金,成立視航生醫的新藥開發公司。
公司所研發的RNA治療眼藥水,
在2021年2月提早通過美國FDA無條件而且提早核准,
執行第一期臨床試驗,
目前已經完成臨床一期試驗,
確認其安全性及有效性,
近期內將會展開下一階段的臨床試驗,
預期順利完成臨床二、三期,取得藥證,
並很快進入近視盛行率極高的亞洲市場。

-
ABOUTDREAMHAWK VISION公司簡介

睛航生物醫學股份有限公司(睛航生醫)
卓夙航教授與梁中玲醫師兩位創辦人於2021年4月成立了睛航生醫,
以RNA治療的技術平台,
積極研發核酸藥物用於治療近視以外的其他眼睛疾病。
SHJ002核酸藥物眼藥水,
於治療乾眼症已經在澳洲、臺灣、泰國展開臨床二期試驗,
並且有國際大藥廠積極尋求授權。
我們的願景目標是成為全球最具創造力以及有價值的眼科新藥生技公司。

-
FOUNDER
INTRODUCTION創辦人介紹 
曾在美國哈佛大學附設醫院接受兒童視光訓練,
目前是教育部部定副教授,也是國家視光師考試命題委員,
是國內外知名的醫師科學家。
梁醫師畢業於長庚大學,
是長庚大學第一位女眼科醫師獲得長庚大學博士學位。
曾經擔任過台北長庚眼科主治醫師,高雄市立聯合醫院眼科主任,
亞洲大學附屬醫院創院眼科主任,目前任職於亮晶晶眼科診所院長。

- 梁中玲博士
卓教授是國際知名的人類基因學專家,畢業於台灣高雄醫學大學醫學系,
並且接受臨床訓練,1992年取得神經內科專科醫師執照。
之後赴美求學分別取得哈佛大學碩士,約翰霍普金斯大學博士,
並曾經在美國國家衛生研究院(NIH)以及洛克菲勒大學擔任博士後研究員,
之後於美國長春藤名校之一的哥倫比亞大學擔任副教授。
卓教授於2005年返台協助母校高雄醫學大學發展基因醫學。
目前是台中中國醫藥大學之特聘教授。
卓教授發表超過190篇國際學術論文,
論文被引用次數超過7200次,並榮獲國內外多次獎項。
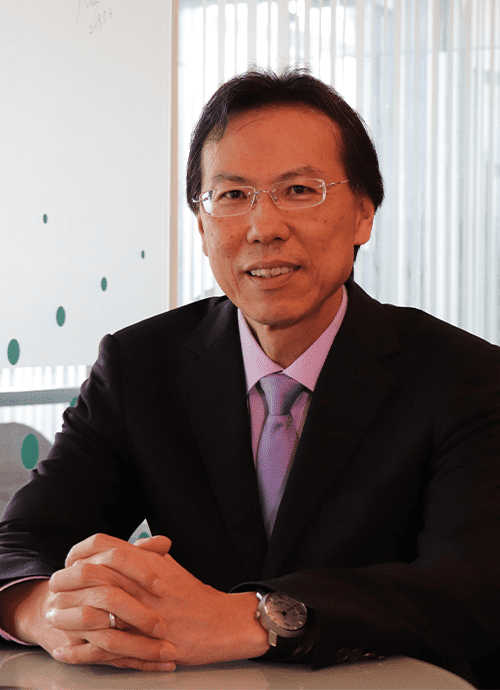
- 卓夙航特聘教授

-
AWARDS得獎與榮譽事蹟














-
RESEARCH相關學術研究


-
近視相關研究點選more可觀看推薦論文more+

-
乾眼症相關研究點選more可觀看推薦論文more+


